Liquid Crystals
Liquid crystals are materials presenting at least one intermediate phase between isotropic liquid and crystalline solid phases. Research in liquid crystals is of great interest from both the fundamental and technologic points of view. Tha later includes applications in fields such as optics, optoelectronics or even medicine
2. Classification of liquid crystals
3. Mesophases
4. Phase transitions
5. Molecular dynamics
6. Dimers and Bananas
7. Confinement
1. What's a liquid crystal?
A liquid crystal is a material that shows one or more intermediate phases between the liquid isotropic and the crystalline solid ones. This intermediate phases or mesophases (so the name "mesogen" for a liquid crystal) appear in function of temperature and/or the concentration in some solvent. In the mesophases, molecules are positionally disordered, but they present an orientational order. This way, these are anisotropic fluid phases. Mesogenic molecules have a very particular geometry which allows the formation of such intermediate phases.
2. Classification of liquid crystals
We shall distinguish between two different types of liquid crystals: thermotropic and lyotropic liquid crystals. In thermotropics, the phase diagrams is just temperature-dependent. the phase diagrams of lyotropics also depends on the compoud concentration in some solvent (water, usually).
The most convencional thermotropics, have uniaxial molecular geometries, presenting a uniaxial rigid unit, that can be rod-like (calamitic liquid crystals) or disk-like (discotic liquid crystals). Attached to these rigid units, there are some terminal flexible chains, providing fluidity to the compounds. The rigid units are usually formed by polar groups, being the molecules polar. In some particular cases, molecular dipolar moments can get coupled, giving rise to ferro., ferri. or antiferroelectric mesophases where there exists a spontaneous polarization.
Calamitic and discotic molecules
All these peculiar properties derive in a wide range of applicabilities. The possibility of quite easily aligning the molecules under the application of external fields (electric, magnetic, surface interactions...), thus changing the direction of the mesophases' main axes, La complementariedad de todas estas propiedades se pone claramente de manifiesto a la hora de enfocar las aplicaciones tecnológicas de estos materiales, can be used in electro-optic devices, such as LC Displays or electrooptic switchers.
Lyotropic molecules are amfifilic, with a polar group that can be water-dissolved and with an apolar hydrofobous chain. Many organic molecules are lyotropic, indeed. Cell membranes pack forming a lyotropic mesophase. Such biocompatibility opens wide the medical and biotechnological applicabilities of lyotropic mesogens: drugs, biologic sensors, virus detectors, food colorants and stabilizers... Some other possibilities also include textile and soap industries.
3. Mesophases
The simplest calamitic phase is the so called nematic (N), in which the molecules are positionally totally disordered, but arrange in a same preferent direction, defined by the director vector, n. A higher degree of positional order arises when molecules are distributed in layers (esmetic layers). Inside the layers, the local order is nematic-like. These are the smectic (Sm) phases.When molecules are perpendicular to the layers the phase is called esmectic A (SmA), but if the molecules are tilted relative to the layers we can find the smectic C (SmC) phase.
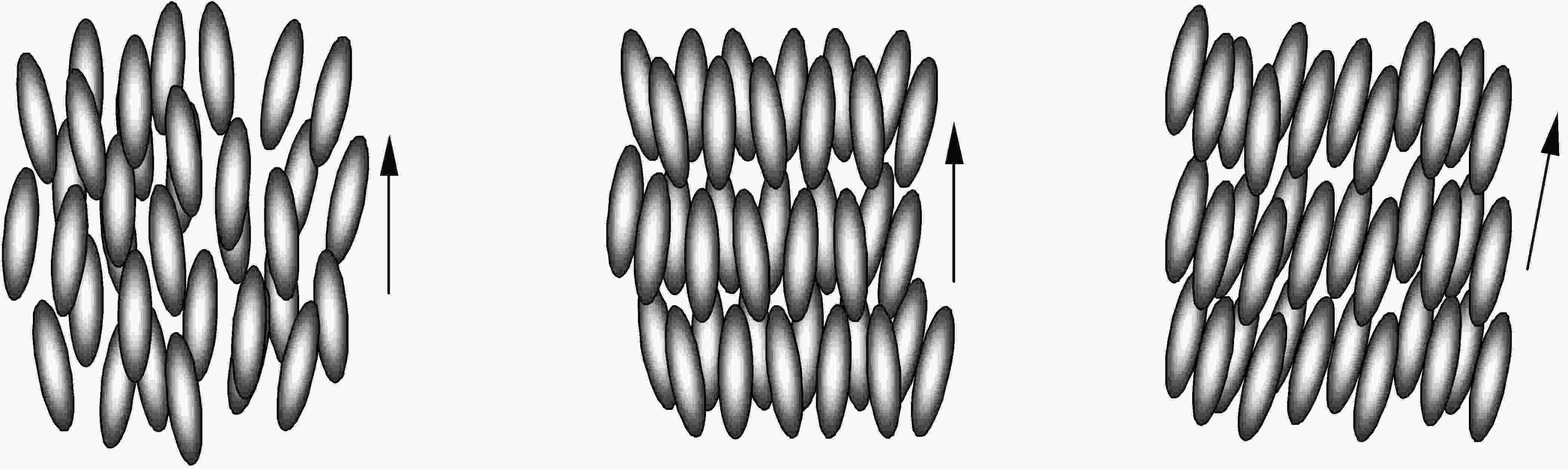
Nematic, Smectic A and Smectic C phases
With respect to the discotic mesophases, the most common are the nematic (ND) and the columnar phases: hexagonal (Ch) and rectangular (Cr), depending on the shape of the unit cell.
Most simple lyotropic mesophases are the interlayed smectic A, as in cell membranes, or the micelle-like ones, tipic of colloidal dispersions.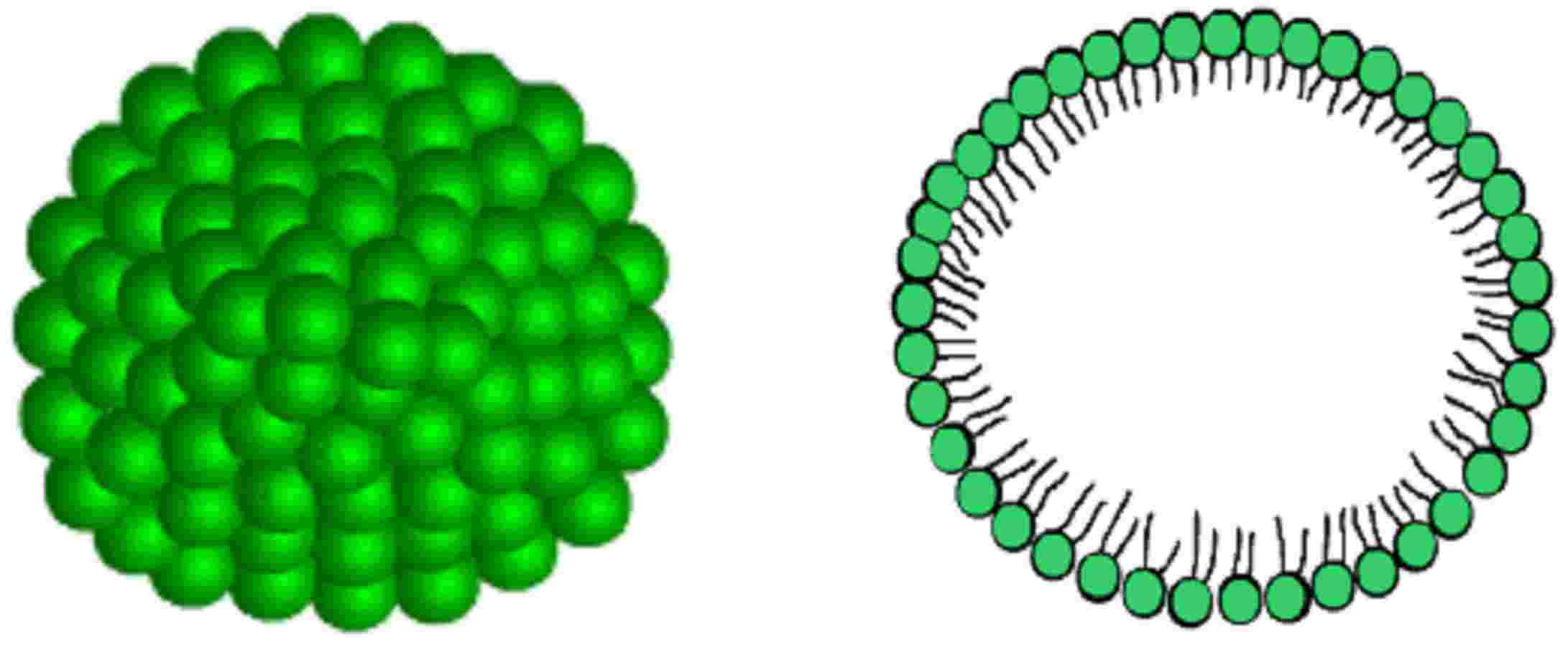
Micellar phases
4. Phase transitions
Liquid crystals exhibit a great variety of phase transitions. These are usually weakly first order (enthalpy change is very small) or second order (critical). Our studies focus basically in the nematic-isotropic (N-I) and the smectic A-nematic (SmA-N) phase transitions.
According to the Maier-Saupe theory the N-I transiton results from competition between thermally excited forces which tend to destroy the orientational order, and molecular-field forces tending to align the molecular axes. This phase transition shall be first-order in nature, but due to fluctuations of the nematic order parameter, the N-I transition could become second-order.
SmA-N phase transition depends on both the nematic and the smectic order parameters and so, its treatment is different. According to McMillan, Kobayashi and de Gennes, the order of this transition depends on the nematic range. When this is small, there exists a relevant coupling between both order parameters, and the phase transition is first-order in nature. Nevertheless, as long as the nematic range gets larger, such coupling changes, driving the order of the transition to secon-order. When the nematic range is large enough, the nematic phses saturates and the phase transition belongs to the 3D-XY universality class.
In contrast, there is another theory by Halperin, Lubensky and Ma, suggesting that the SmA-N phase transition is always weakly first-order in nature.
5. Molecular dynamics
Due to their molecular structure, polar liquid crystals show a very interesting dielectric spectrum, which reflects the molecular dynamics. The theory of Nordio, Rigatti and Segre for calamitic molecules, sais that there are for relaxational modes, which are: the molecular reorientations around their large (mode 1) and short (mode 2) axes, when they are aligned parallel to the applied electric field, as well as a precession around the molecular director (mode 3) and a rotation around the short axis of the precessing molecules (mode 4), when they are perpendicular to the field.In more complex molecules additional molecular or intramlecular modes could appear.
6. Dímers and Bananas
Besides from conventional calamitic and discotic liquid cryatals there is a great variety mesogens with other possible molecular geometries. A couple of these are liquid crystal dimers and bananas:
Dimers
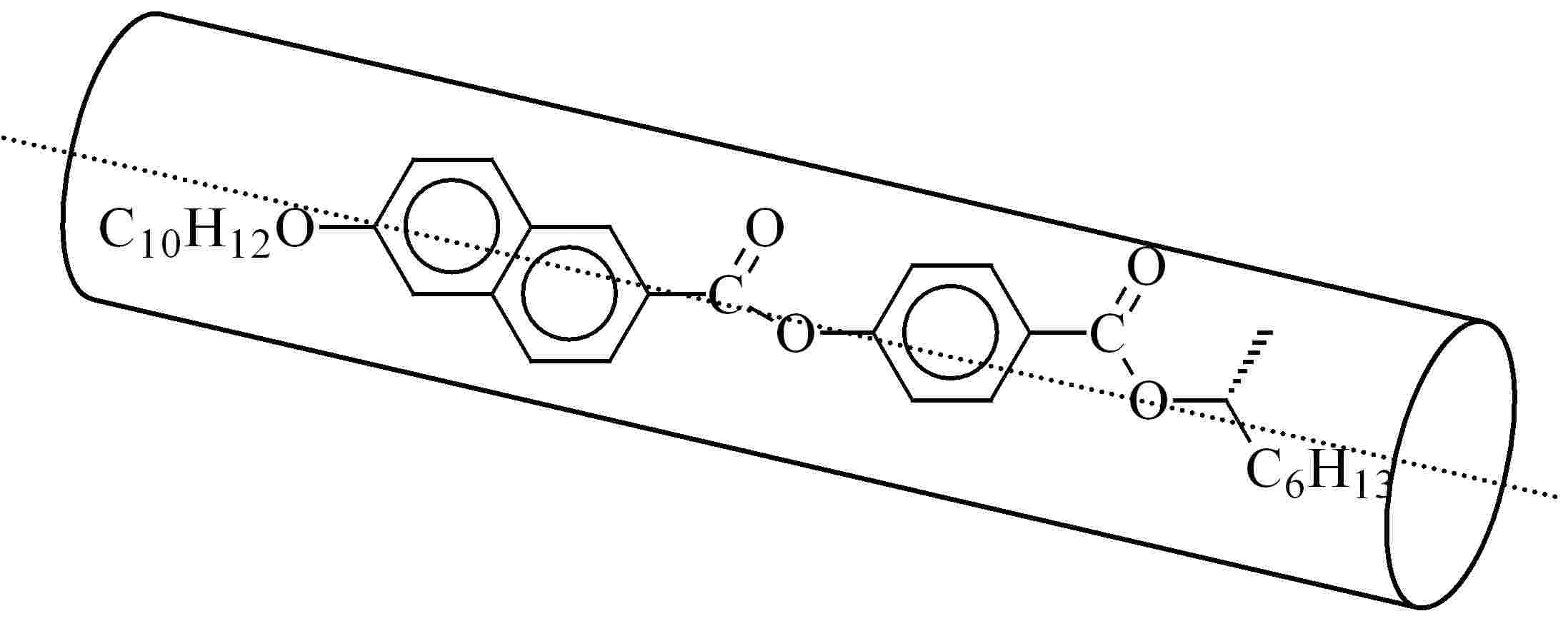
These compounds are formed by two rigid mesogenic units linked by a flexible chain. Depending on the nature of the rigid groups and the flexible spacers, as well as on their relative orientations, several types of dimers can be distinguished.
Extended and bent conformations of an even dimer
The introduction of flexible units between mesogenic groups induces the apparition of additional modes in the dielectric response, from which there is possible to obtain information about the molecular dynamics. it is shown to exist a polarization transfer between modes as temperature changes. Such intramolecular transfer can be understood as the existence of internal-external mode-coupling, i. e., as a change in the average moelcular-shape.
Bananas
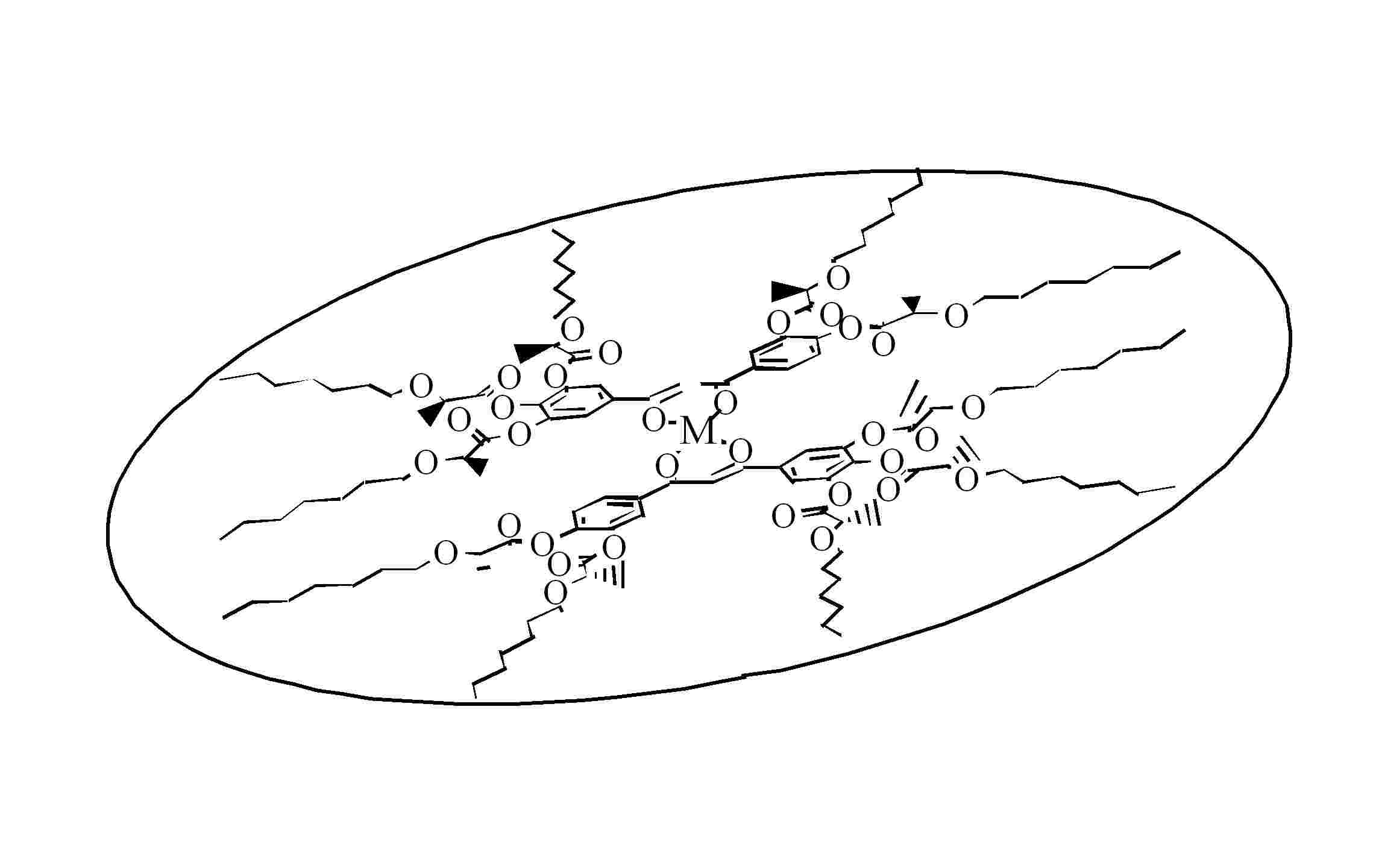
The bent or banana-shaped liquid crystals where first discovered in the 1990's by japanese scintists. They present very peculiar mesophases due to their special molecular geometry, thus being a field of intent activity and research since then. Some mesophases present a molecular arrangement in helicoidal structures, confiring ferroelectric properties to the materials.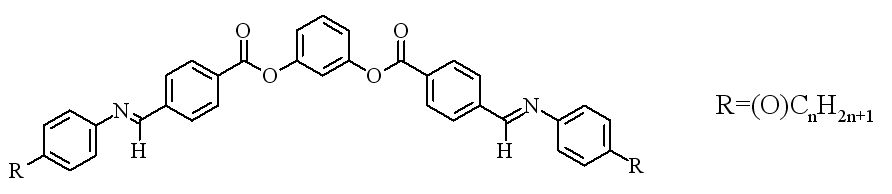
Banana-shaped molecule
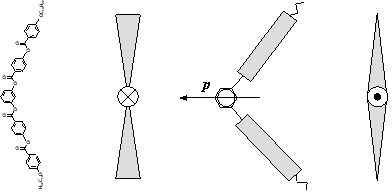
Different views of a banana-shaped molecule
We have obtained some interesting results, produced by the application of electric fields: the induction of ferroelectricity in antiferroelectric compounds and the induction of a B1-B2 phase transition at a fixed temperature.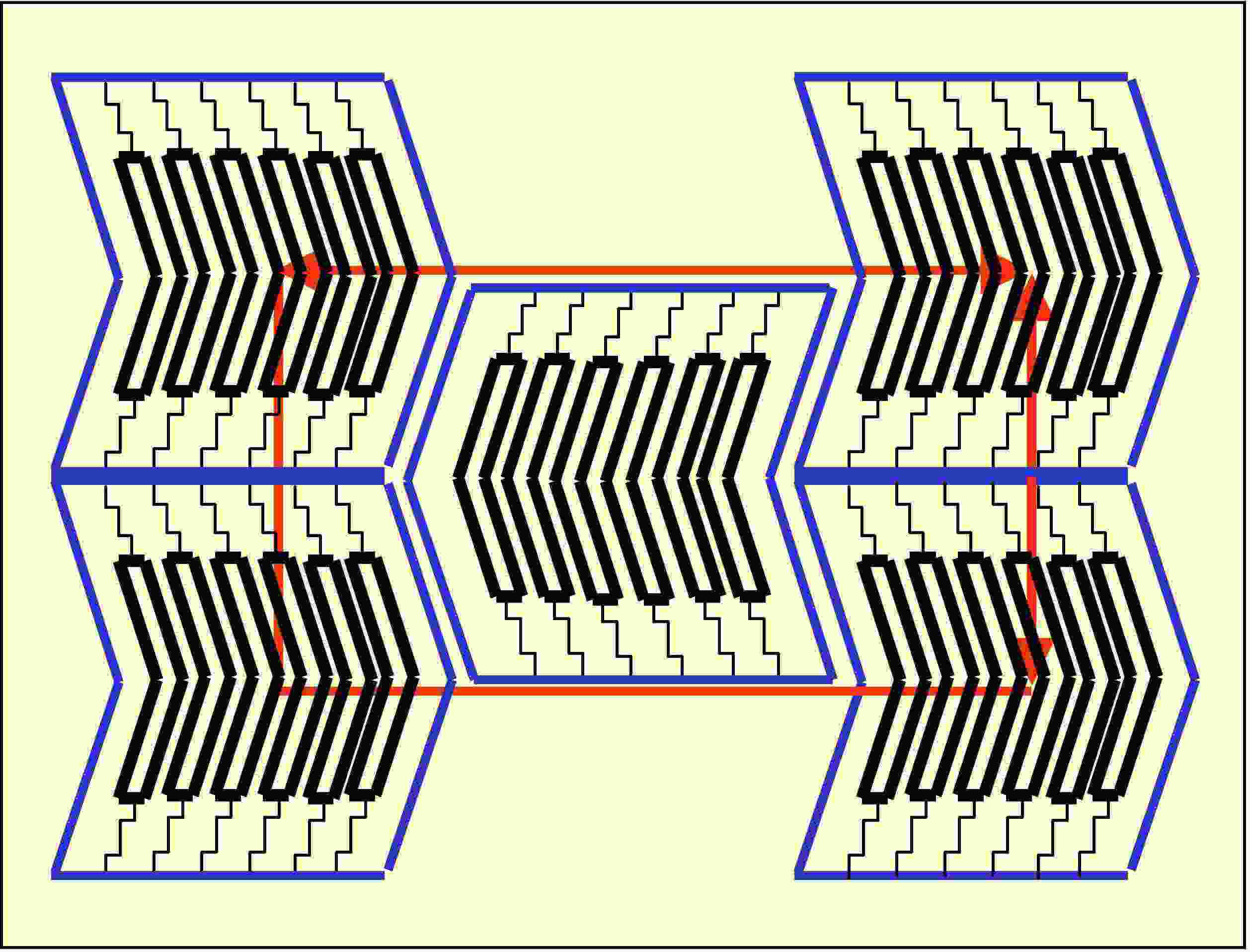
B1 phase
Racemic and Homoquiral Antiferroelectric B2 phases
7. Confinement
Confinement of liquid crystals allows making progress in the study of surface effects on such systems. This study is of great interestr in order to design liquid crystal cells for technologic applications. It is evident that understanding the variation of the physical properties of the confined liquid crystal, with respect to the bulk system, will help as improving not just in the fundamentals of the liquid crystals, but also in the field of design and synthesis of brand new functionalized materials for very particular applications.
Confining liquid crystals we can form complex dielectric structures, as they are usually polar in nature. Complex dielectric systems are dielectric structures in which the dielectric constant can change in a visible-wavelength-like scale, and with a spatially dependent (could be periodic or random) refraction index.
Periodic dielectric structures are called Photonic Crystals or PBG (Photonic Band Gap). First PBGs were produced for microwave wavelengths, but they were quickly adapted to visible waveltength. Their synthesis is, nevertheless, a extremely difficult task, because their geometry needs a very fine periodicity to properly tune theoptical properties of light. Relative to light, PBGs are in fact similar as semiconductors to electrons. Most common photonic crystal is a SiO2 matrix with sferic holes in teh nodes of a FCC cubic structure. Applications of such materials are embedded in the field of photonics: lasers, light control displays, waveguides, filters or cell phones, among others.
SiO2 photonic crystal
Random dielectric structures consist on materials formed by porous structures, with randomly distributed different-shaped holes. If the proper conditions are acomplished, they can be used for the same purpose as photonic crystals. Such conditions mean the possibility of confine some liquid crystal within, so the lack of periodicity can be replaced by the mesogen properties, that allow tuning the light mean free path, for example, or the diffusion constant. A nematic liquid crystal is able to induce some kind of anisotropy in the light mean free path, and this property can be properly tuned by an external field, such as temperature or an applied electric field. Besides, this kind of materials presents two clear advantages respect to photonic crystals: thery are relatively simple to be synthesised and, with respect to applications, more flexible. In contrast, it is essential to know and control how the confinement changes the liquid crystal properties.
CONFINING STRUCTURES
There are many different ways to confine a material. Among all the structures we can find porous matrices where the material is introduced and particles that are dispersed into the material. We are mostly using two types os structures: pseudo-ordered matrices (Anopore) and randomly dispersed particles (Aerosil).
Anopore membranes
Anopore structures are porous membranes, mostly used as chemical filters. The diameter of the almost-parallel cylindrical-shaped cavities are about 200 nm, and the length about 60 micron.

Anopore membrane
Once we have the porous membrane, we treat it chemically in order to functionalize the porous surfaces, so we can obtain different molecular alignments inside them. This functionalization is made by means of a chemical surfactant, and depends on the confined liquid crystal. Surface effects can be modulated by means of the surfactant, and they induce significant changes in the liquid crystal properties. Thus, by treating the membranes with a specific solution of hexadecyltrimethylammonium bromide (HTBA), conventional calamitic molecules tend to align perpendicular to the surfaces. This happens because HTBA molecules are amfifilic, with the polar group attaching to the aluminium oxide of the surfaces and the hydrofobous chain to the terminal flexible chain of the mesogens. Such an aligment allows studying, by dielectric spectroscopy, the molecular dynamics of modes 3 and 4 explained above.
Aerosil particles
"Silica Aerogel" or "Aerosil" is just a group of sferic silica particles, of about 7 nm diameter, with hydrophilic or hydrophobic units in the surface. By dispersing these nanoparticles into a mesogenic material, a new type of confinement is produced, depending on the solution concentration. If Aerosil density in the solution is above some threshold value, an Aerosil network is generated, drastically restricting the system dynamics.
Here is listed our collection of publications on liquid crystals
Share: